Abstract
Background
Subcutaneous bortezomib administration has huge advantages to treat patients with poor venous accesses and, generally, it is convenient for both patients and physicians because it overcomes problems related to a prolonged intravenous infusion or the insertion of a long-term central venous access device. Moreover, overall incidence of peripheral neuropathy is lower with the subcutaneous administration in comparison with the intravenous route, reducing the possibility of a therapy discontinuation related to this adverse event. In recent years, for some non-histotoxic anti-cancer drugs, such as rituximab, trastuzumab or cladribine, the subcutaneous route, as alternative to the intravenous one, has been successfully compared and the possibility of a self-administration modality for adequately informed patients or adult care-givers was also demonstrated. Previous randomized trials proved that subcutaneous bortezomib administration is pharmacologically and clinically equivalent to the intravenous one, with an equal safety profile.
Aims
We focused our retrospective study on a population of elderly patients not eligible for high-dose therapy with autologous hematopoietic stem-cell transplantation (HDT-HSCT) as frontline therapy, but treated with VMP regimen, that was demonstrated to be highly effective in this setting.
Methods
Since 2009, in our Hematology Unit, 63 patients requiring bortezomib for the treatment of MM, in association with orally administered prednisone and melphalan (VMP), performed subcutaneous injection of bortezomib at home for personal or logistic reasons. Initially, the drug was administered by qualified personnel; subsequently, the patient or an adult care-giver learned to inject it in the deltoid muscle. Patient were supplied with bortezomib in ready-to-use plastic syringes, where the drug was appropriately constituted in saline solution, under hood in sterile conditions by qualified personnel.
Patients received 4-weeks cycles of melphalan (9 mg/m2) and prednisone (60 mg/m2) on days 1 to 4, bortezomib (1.0 mg/m2) on days 1, 8, 15 and 22 (mean number of cycles 9, r. 3-12), according to a schedule adapted for frail patients [4,9,10]. The first cycle was usually administered at hospital to assess and confirm the safety of this route.
Results
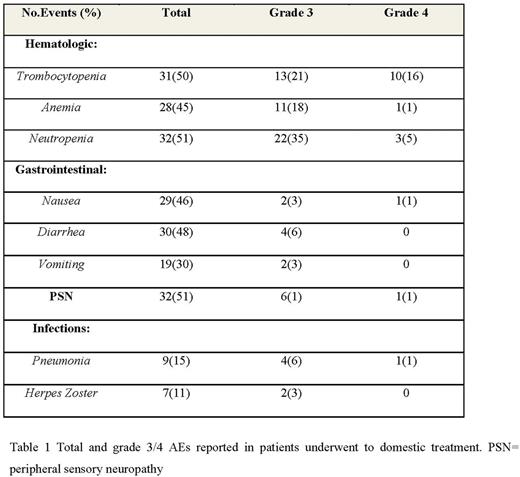
Data about efficacy and safety were similar to those observed in major clinical trials. We evaluated the overall response rate (ORR): 45 patients (72%) achieved a response; in particular, 20 patients (32%) achieved a complete response (CR), 25 patients (40%) achieved a partial response (PR) and 6 patients (9%) achieved a minimal response (MR) after four cycles of therapy and the adverse events rate (Table 1). Moreover, results showed the equal incidence of adverse events (AEs) for domestic administration (Table 1). There were no severe AEs requiring hospitalization or access to Emergency Unit.
Conclusions
These results confirm the effectiveness and safety of subcutaneous bortezomib, in a real-life-experience, and define a new possibility of safe auto-administration in a comfortable domestic setting. We believe that domestic treatment can significantly improve the quality of life of the patients, avoiding unnecessary transfer to the hospital without reducing treatment efficacy.
Pane: Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal